What can aquarium sealant, fish embryos and immune cells tell researchers about pediatric heart failure?
In terms of data, comparing adult and pediatric heart failure research is like comparing an ocean to a pond. Pediatric heart disease is rare and heterogeneous, which makes it difficult to study. Across several labs at Children’s Hospital Colorado and the Anschutz Medical Campus, teams of researchers, many of them post-doctorate and junior faculty, are investigating the early heart from an ever-growing array of angles.
The need for pediatric heart failure data
Pediatric cardiologist Stephanie Nakano, MD, is gluing a heart cell to a machine. The cell came from a tissue bank of diseased hearts taken, with consent, from nearly every patient who gets a heart transplant at Children’s Colorado. The glue is regular old aquarium sealant.
Dr. Nakano first isolates the individual heart muscle cells. Under a microscope, she can identify the proteins that make them contact. Then she aligns the cell with the needle tips of the machine and pilots a pair of motorized micropositioners to either end of the cell. She’ll need to make it happen within three minutes, before the glue starts to cure.
“It’s a pretty delicate technique,” she says. “It took me a long time to learn.”
Once attached, the machine will measure the precise amount of contractile force this particular cell can generate. The technique itself has been around nearly 50 years, and it’s been used to generate a lot of mechanical data about adult heart cells, giving rise to treatments that have led to vastly better outcomes in many forms of adult heart failure.
But those medications don’t work as well in kids, even for the same conditions.
Similarities and differences in adult and pediatric heart failure phenotypes
Comparing the cells of kids with dilated cardiomyopathy with the literature on their adult counterparts, Dr. Nakano, along with her mentor, pediatric cardiologist Shelley Miyamoto, MD, is making progress understanding why.
“We found some similarities between pediatric and adult patients in the failing phenotype, but also differences,” Dr. Nakano says. “The amount of calcium it takes to make the cells contract is similar, but the amount of cooperativity between these filament proteins is less, meaning it’s more difficult for the necessary proteins to bind. That hints at differences at the protein level.”
Understanding those differences could eventually help researchers develop new drugs, or figure out ways to tweak existing drugs, to increase their effectiveness in kids. It’s just one way into the deep and complex web of problems that can affect a child’s heart.
“We’re trying to understand these problems, but we’re also investing in these researchers,” Dr. Miyamoto says. “I was a junior researcher when we started this whole thing, and the idea is that it’s constant turnover and growth. Leadership evolves. Projects evolve. Different labs branch off and become independent. It’s complementary and collaborative.”
That evolution has led to a diverse array of research pursuits. Like one that involves an entirely different kind of evolution: our shared ancestry with fish.
Tracking the lateral plate mesoderm
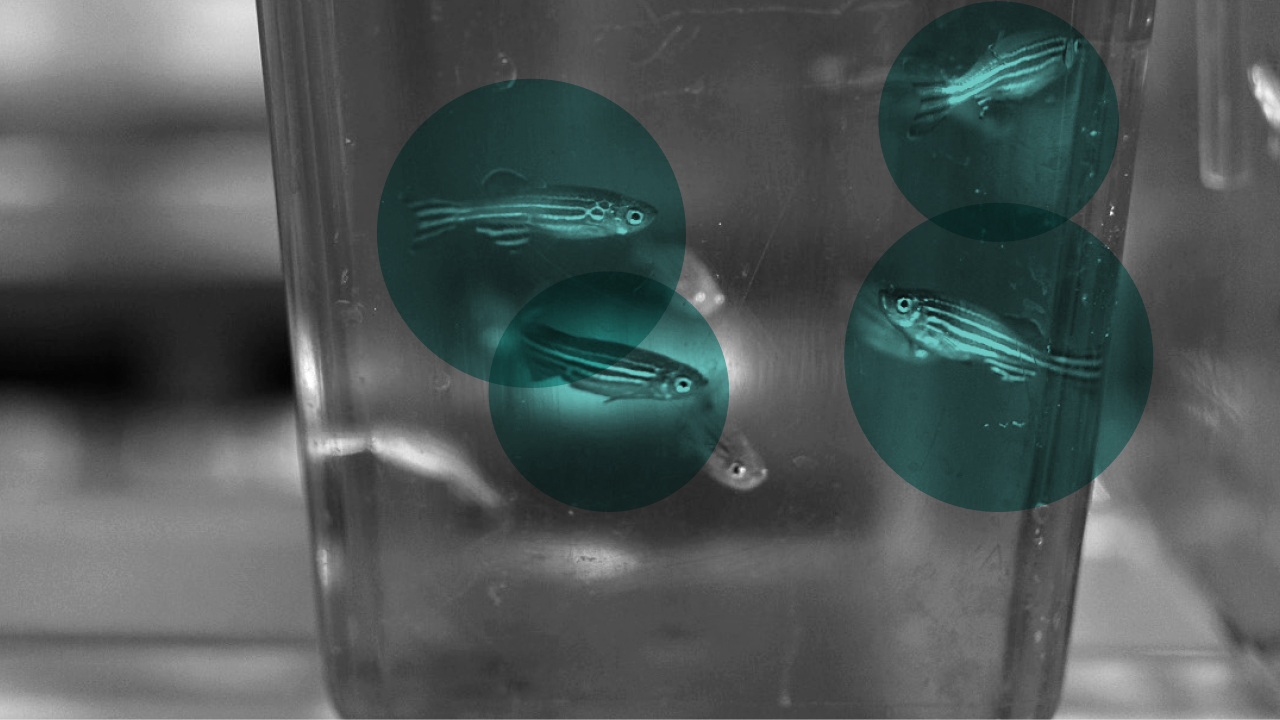
Several floors down, the team of molecular biologist Christian Mosimann, PhD, is injecting DNA into the embryos of zebrafish. The DNA will make them glow in the dark.
“They tolerate it really well,” he says. “I don’t think they know they’re glowing.”
Fish, like all vertebrates, have a lot of genetic material in common with humans, and their cardiovascular systems work in much the same way, with a centralized heart that pumps blood to deliver oxygen and other nutrients through the body.
Dr. Mosimann and his research group are interested in how that system develops.
“Through genome sequencing, we can pinpoint the changes patients with cardiovascular anomalies have in their genome,” he says. “And with CRISPR technology we can then introduce those mutations in zebrafish and see, in great detail, what effect these changes have on cardiovascular development.”
That’s possible through Dr. Mosimann and his lab’s work uncovering the first genetic means to track the lateral plate mesoderm, or LPM, the group of cells that go on to build the heart and its associated organs.
“These systems reflect what heart and circulatory systems looked like millions of years ago, when we were not even fish yet,” says Dr. Mosimann, “but something much more primitive.”
Color coding the development of the cardiovascular system
Having identified the proteins that regulate the LPM’s emergence in the embryo, Dr. Mosimann’s lab can label the earliest heart-forming cells with transgenic markers that color-code their parts. It works in several different animal models, but it’s especially useful in zebrafish, because you can see right through them.
The discoveries are intriguing. LPM cells go on not only to build the heart and circulatory system, but also to build disparate systems further flung: bones in the upper extremities, muscles in the neck and face. That insight has put previously clinical diagnoses like Holt-Oram syndrome, characterized by heart problems and anomalies in the hands, in new context. Both heart and hand issues are linked in early development and correlate with a mutation of the gene TBX5. Indeed: TBX5 mutations in zebrafish result in heart problems and missing pectoral fins.
“That’s the big picture,” says Dr. Mosimann. “If we can model complex, connected heart disease in fish and learn what phenotypes we need to pay attention to, then maybe eventually we can say, ‘Well, the patient has this mutation; fish with the same mutation have problems with their joints and kidneys too.’ And our clinician colleagues somewhere down the road can make those connections and make a care plan that improves that kid’s quality of life.”
Lateral plate mesoderm genetic programming and environmental factors
Aside from the cardiovascular system, the lateral plate mesoderm also gives rise to mesothelial cells, which provide a sort of protective shrink wrap for organs and produce mucous to lubricate them. Dr. Mosimann’s team isolated the first genetic program that sets them apart from other cells in the LPM. It’s the same program they use to repair themselves, after, say, a surgical incision.
And in the case of mesothelioma, a type of lung cancer triggered by asbestos fibers, Dr. Mosimann’s team found that it’s that same genetic program that goes awry.
“It’s just an example of a way we can connect developmental biology to environmental factors, in this case asbestos exposure,” says Dr. Mosimann. “It’s a bigger story than just glow-in-the-dark fish.”
Developing biomarkers for pediatric heart failure
As clinicians, one of the most vexing problems Drs. Miyamoto and Nakano encounter in their patients is the unpredictable heterogeneity of outcomes. Some kids with, say, single ventricle heart disease do really well. With the help of three well-established operations that reconfigure their circulation, they can live long, relatively healthy lives.
Others’ hearts’ fail. That’s a growing public health problem, particularly as better treatments lead to longer survival for kids with serious heart anomalies. A transplant might last 15 years. For a 2-year-old, that’s a lot of heart transplants down the road.
A much better solution would be to preserve the existing heart, but that’s a complicated problem to figure out. In the adult world, for example, many studies are done with biopsies. They’re relatively routine. For kids, whose hearts are much smaller, they’re intolerably risky. Dr. Miyamoto’s researchers get around that working with explanted tissues. But they’re also increasingly looking at the blood.
“If we could predict who would do poorly that could help inform treatment,” Dr. Miyamoto says. “We’re trying to find cells and other biomarkers that can do that, but we need to be able to get them from a blood draw.”
Cataloguing peripheral blood mononuclear cells
As she transitions from Dr. Miyamoto’s lab to independent research, Dr. Nakano is currently focused on those circulating biomarkers. In fact, last year she identified a set of circulating microRNAs that predicted survival or either transplant or death by 1 year of age.
She’s now investigating the potential role of the immune system through rigorous classification of the peripheral blood mononuclear cells, or PBMCs, in the blood of single ventricle patients on their way to surgery. Collaborating with the Human Immune Monitoring Shared Resource, or HIMSR Core, a collaborative immunology lab on the Anschutz Campus, Dr. Nakano is working to define the types of immune cells in circulation, their activation status and what kind of cytokines they may produce.
“In order to really figure out if a cell type works as a marker, if it’s a good surrogate for what’s happening in the heart,” she says, “you need to know exactly what you’re working with.”
And if that doesn’t work out, well, there are plenty of other fish in the sea.
Featured Researchers
Stephanie Nakano, MD
Advanced heart failure and transplant cardiologist
The Heart Institute
Children's Hospital Colorado
Associate professor
Pediatrics-Cardiology
University of Colorado School of Medicine

Shelley Miyamoto, MD
Chair of Pediatric Cardiology, Co-Director of the Heart Institute
Children's Hospital Colorado
Section Head of Cardiology, Department of Pediatrics
Pediatric Cardiology
University of Colorado School of Medicine
Christian Mosimann, PhD
Associate professor
Pediatrics-Developmental Biology
University of Colorado School of Medicine





 720-777-0123
720-777-0123