Key takeaways
-
This study compared outcomes of routine in-utero treatment with steroids to manage NLE to patients who were mostly untreated.
-
Transplacental fetal treatment (TFTX) with steroids improved survival and decreased dilated cardiomyopathy (DCM) prevalence.
-
The use of steroid TFTX contradicts current recommendations to solely observe, which may have future clinical implications.
Background: A lack of consensus exists about prenatal neonatal lupus erythematous treatment
Neonatal lupus erythematosus (NLE) is a spectrum of maternal antibody-mediated fetal and early childhood disorders with underlying cardiac manifestations. It is caused by high titers of maternal anti-Ro antibodies that cross the placenta, enter the fetal bloodstream and bind to fetal proteins, triggering a cascade of inflammatory responses in the fetus. Fibrosis from resulting inflamed fetal heart tissue can manifest as:
Atrioventricular block (AVB)
- 3° AVB
- Most common
- Develops between 18 and 24 weeks of gestation
- Significant risk of mortality
- Causes bradycardia
- May have concomitant myocardial injury
- 1° AVB and 2° AVB
- May progress to 3° AVB
- Sinus node disease, which may coexist with 3° AVB
- Endocardial fibroelastosis (EFE), which may coexist with 3° AVB
- Dilated cardiomyopathy (DCM)
Other disorders associated with increased perinatal mortality, which may or may not present with AVB, include:
- Earlier gestational age at diagnosis
- Ventricular heart rate (HR) ≤50 beats per minute (bpm)
- Hydrops
- Ventricular dysfunction
- Prematurity
5% to 30% of children born with 3° AVB will develop DCM with a potential need for:
- Heart failure treatment
- Cardiac transplantation
No current consensus exists on optimal prenatal management of NLE or the use of anti-inflammatory medications. Some centers offer transplacental fetal treatment (TFTX) with steroids.
This multicenter study, including the Fetal Cardiology Program within the Colorado Fetal Care Center at Children's Hospital Colorado, examined the impact of TFTX with steroids on markers of cardiac neonatal lupus (C-NL) manifestations. Researchers compared outcomes of fetuses diagnosed with advanced AVB and treated with dexamethasone with or without intravenous immune globulin with untreated fetuses.
Methods: characterization and comparison of fetuses diagnosed with cardiac NLE
Study populations
Study population includes all fetuses diagnosed with cardiac NL before July 1, 2019, whose mothers received the following recommended treatment protocol:
- 4- to- 8mg/d dexamethasone upon C-NL diagnosis with weaning during the third trimester.
- Add β-stimulation with 30-40 mg/d salbutamol or 5-30 mg/d terbutaline for HR below 50 to 55 bpm or ventricular dysfunction.
- Add 1g/kg or 70g/dose of intravenous immune globulin (IVIG): one dose every 2–3 weeks until birth; 2 g/kg at birth for extensive EFE or incomplete AVB. If extensive FFE or incomplete AVB persisted, some sites delivered 1 mg/kg per dose twice daily prednisone until 8 weeks after birth.
A stress dose of steroids was given to mothers at delivery and then they were slowly weaned off.
Study exclusions
- 7 elected pregnancy termination without TFTX
- 3 declined prenatal steroids
- 6 received steroids only later in gestation
- 5 were not offered steroids
- Anti-Ro antibody-negative pregnancies with fetal AVB
Research protocol
Detailed fetal echograms were obtained and scored using the following parameters:
- AVB
- 1° AVB: 1:1 AV conduction with AV prolongation z score greater than 6 using the normal mean for gestation age
- 2° AVB: atrial impulses not conducted to ventricles
- 3° AVB: a complete absence of AV conduction
- 2° to 3° AVB: episodes of both 2° AVB and 3° AVB
- Hydrops
- Effusion in at least two fetal body compartments
- EFE
- Abnormal echogenicity on endocardial surfaces of the chambers and/or valve leaflets
- DCM:
- Postnatal left ventricular (LV) dysfunction
- Ejection fraction less than 50%
- LV dilation
- Postnatal left ventricular (LV) dysfunction
Outcomes of treated patients through December 31, 2019, were compared to primarily untreated fetuses with antibody-mediated 2° and 3° AVB published since 2007.
Results: better outcomes from in-utero treatment of C-NL versus inconsistently treated

Transplacental treatment
A daily dose of dexamethasone was administered within one week of diagnosis.
- 59% received 8 mg
- 1.5% received 6 mg
- 39% received 4 mg
- 0.7% received 2 mg
Additional maternal treatment
- 36% received sympathomimetics
- 47% received IVIG
Dosage adjustments
- 32 maintained (mainly at 4 mg/d)
- 87 reduced (mainly to 2 mg/d)
- 11 stopped before delivery
- 2 lowered (due to maternal side effects)
Fetal growth restriction (FGR) among treated fetuses was documented in 39 of 125 live births. Compared to normally grown newborns, FGR was associated with earlier C-NL diagnosis, longer duration of treatment, and higher amount of prenatal exposure to dexamethasone. There were no significant correlations with 3° AVB versus. other diagnoses or differences in gestational age at delivery.
Outcomes by diagnosis
3° AVB
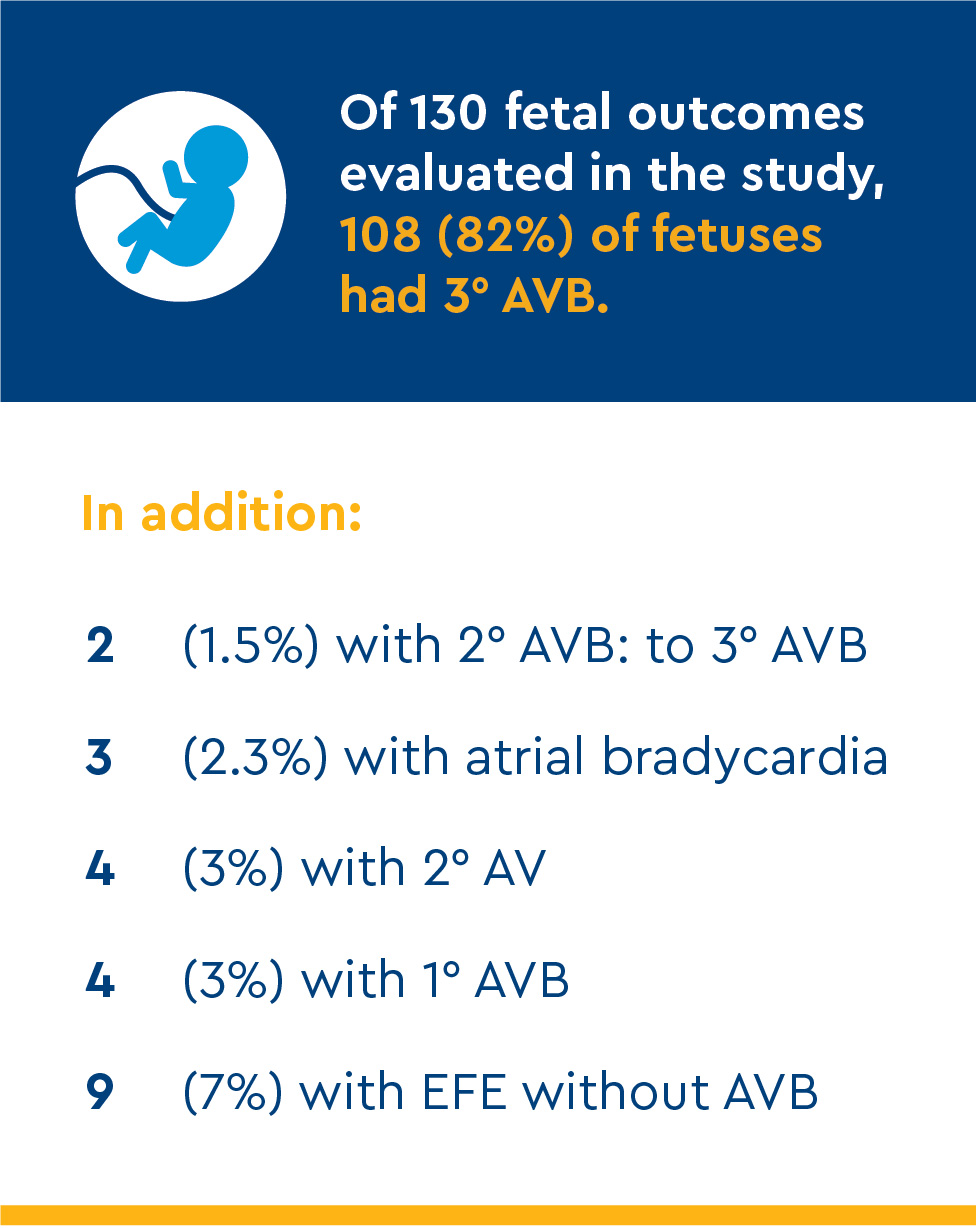
Mortality of 108 initial fetal cases:
- 1 termination at 23 gestational weeks for maternal eclampsia
- 4 in-utero deaths between 23.4 and 29 weeks
- 3 neonatal deaths
Perinatal death variables:
- Atrial rate <90 beats/ minute
- Endocardial fibroelastosis
- Ventricular dysfunction
- Ventricular rate <45 beats/minute
At a 5.9-year follow-up of 100 neonatal survivors:
- 85 were paced
- 97 had normal LV function at follow-up
- 2 had mild LV dysfunction
- 1 had severe cardiac dysfunction and needed a heart transplant
- 2 had 3° AVB with EFE had spontaneous tricuspid or mitral valvular chordal rupture
Incomplete AVB
All 10 incomplete AVB cases survived with normal cardiac function.
Atrial bradycardia or standstill
Two fetuses were observed with atrial bradycardia of 84 and 90 bpm without AVB. TFTX did not improve atrial rates.
Isolated endocardial fibroelastosis (EFE)
Nine fetuses had EFE without AVB or sinus node disease. The EFE affected the ventricular and atrial walls, papillary muscles and/or perivalvar tissues. All remain alive, with normal cardiac function and normal 1:1 atrioventricular conduction.
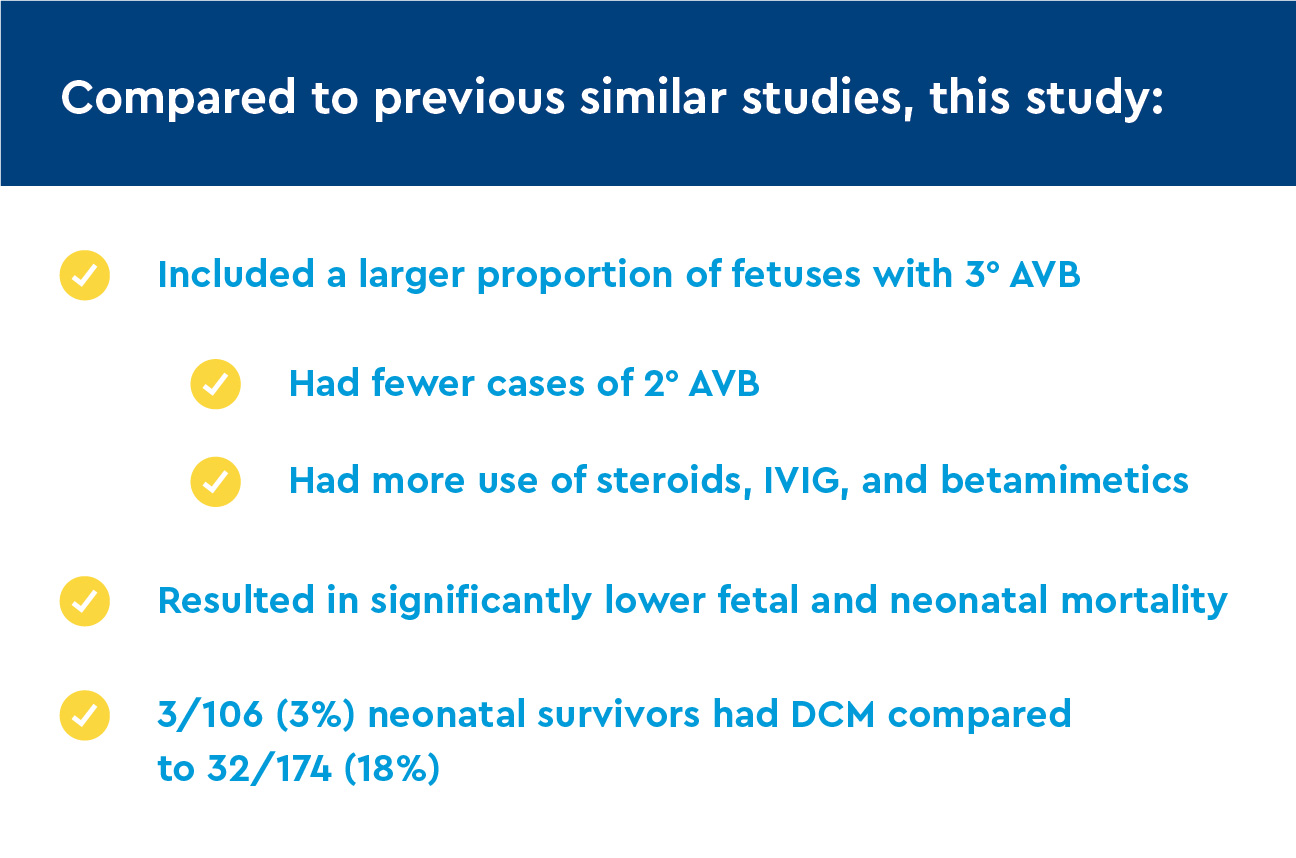
Comparison of findings to previous studies
Previous studies and results from the study authors showed better treatment outcomes for immune-mediated C-NL using TFTX steroids. The American Heart Association recommends observation without the use of prenatal steroids as the most useful treatment for AVB. When compared to immune-mediated AVB, idiopathic/genetic forms of heart block are:
- Incomplete and transient
- Steroid-treatment resistant
- Have a better prognosis
Compared with mostly untreated patient cohorts, there was a lower presence of postnatal DCM. There was one case of 3° AVB with severe postnatal cardiac dysfunction.
Discussion: In-utero treatment for C-NL improves survival and decreases the risk of DCM
Pathological features of Cardiac NLE
In postmortem studies of patients with antibody-positive AVB, cardiac involvement and immunoglobulin G deposition extended beyond the electrical conduction system.
Prenatal diagnosis of C-NL and Outcome Determinants
In fetuses with advanced AVB:
- 40% had abnormal cardiac echogenicity
- 10% had ventricular dysfunction
- 30% had pericardial effusion
- 7 with 3° AVB died in-utero or as neonates:
- Had at least two risk factors for mortality
- Had the lowest atrial (100% mortality) or ventricular HR (40% mortality)
In fetuses without 3° AVB:
- 17% had cardiac manifestations without 3° AVB
- 4/6 fetuses with 2° AVB or 2° AVB to 3° AVB developed 3° AVB
Treatment-related serious adverse effects are rare. Prolonged prenatal steroid exposure contributes to IUGR but does not affect delivery age or postnatal survival.

Conclusion: low risk of perinatal mortality, postnatal cardiomyopathy with TFTX
Study findings indicate TFTX, including routine use of steroids, improved the survival of fetuses with cardiac NLE and reduced the postnatal prevalence of DCM when compared with previously published reports.
Postnatal DCM appears relative to the degree of fetal myocardial inflammation, not the use of ventricular pacing. Survival is expected unless there are poor prognostic factors present at diagnosis.





 720-777-0123
720-777-0123










