Key takeaways
-
TRK inhibition may provide some objective clinical effectiveness in DMG patients with the NTRK mutation.
-
Previously, safety concerns often prevented diagnostic biopsies for patients with DIPG.
-
New data supports that biopsies are safe when performed by an experienced neurosurgeon.
-
It is important to identify the small subset of patients who may benefit from available targeted therapies.
Background: potential of TRK inhibitors in DMG with NTRK gene mutations

Diffuse intrinsic pontine gliomas (DIPG) are almost always fatal and there are no effective therapies for the disease. Radiation therapy can provide temporary relief, but fewer than 10% of children live beyond 2 years after diagnosis.
Chromosomal translocation mutations involving the group of NTRK genes have been found in several adult and pediatric cancers. These mutations result in abnormal proteins which remain inappropriately “turned on,” driving abnormal cell differentiation and cancer growth.
The TRK inhibitors larotrectinib and entrectinib now have FDA approval for all cancers with abnormal TRK fusion proteins. Patients with high-grade gliomas and NTRK fusion proteins were not included in the original clinical trials for these drugs, however. Studies have demonstrated these drugs' efficacy in high-grade gliomas without H3K27M mutations, but the potential role of TRK inhibitors in patients with DMGs positive for the TRK fusion protein has not been investigated.
In this study, Nathan Dahl, MD, and other investigators reported the clinical characteristics of three TRK fusion-positive children with DMGs, examined the transcriptional relevance of NTRK fusions in these tumors at a single-cell level, and discussed the therapeutic implications. The study authors included researchers from the Neuro-Oncology Program for Children with Central Nervous System Tumors at Children’s Hospital Colorado.
Methods: tumor specimen analysis from DMG patients positive for the TRK fusion protein
Researchers analyzed human tumor specimens (obtained via diagnostic biopsy or autopsy) from three pediatric patients with DMG. All were identified as positive for the TRK fusion protein via whole genome sequencing or whole transcriptome sequencing (using RNA-seq).
- Single-cell RNA sequencing (scRNAseq) was performed on individual cancer cells and specific transcriptome characteristics were further analyzed. These findings were contrasted with single-cell RNA sequencing from two DMG samples not positive for the TRK fusion protein.
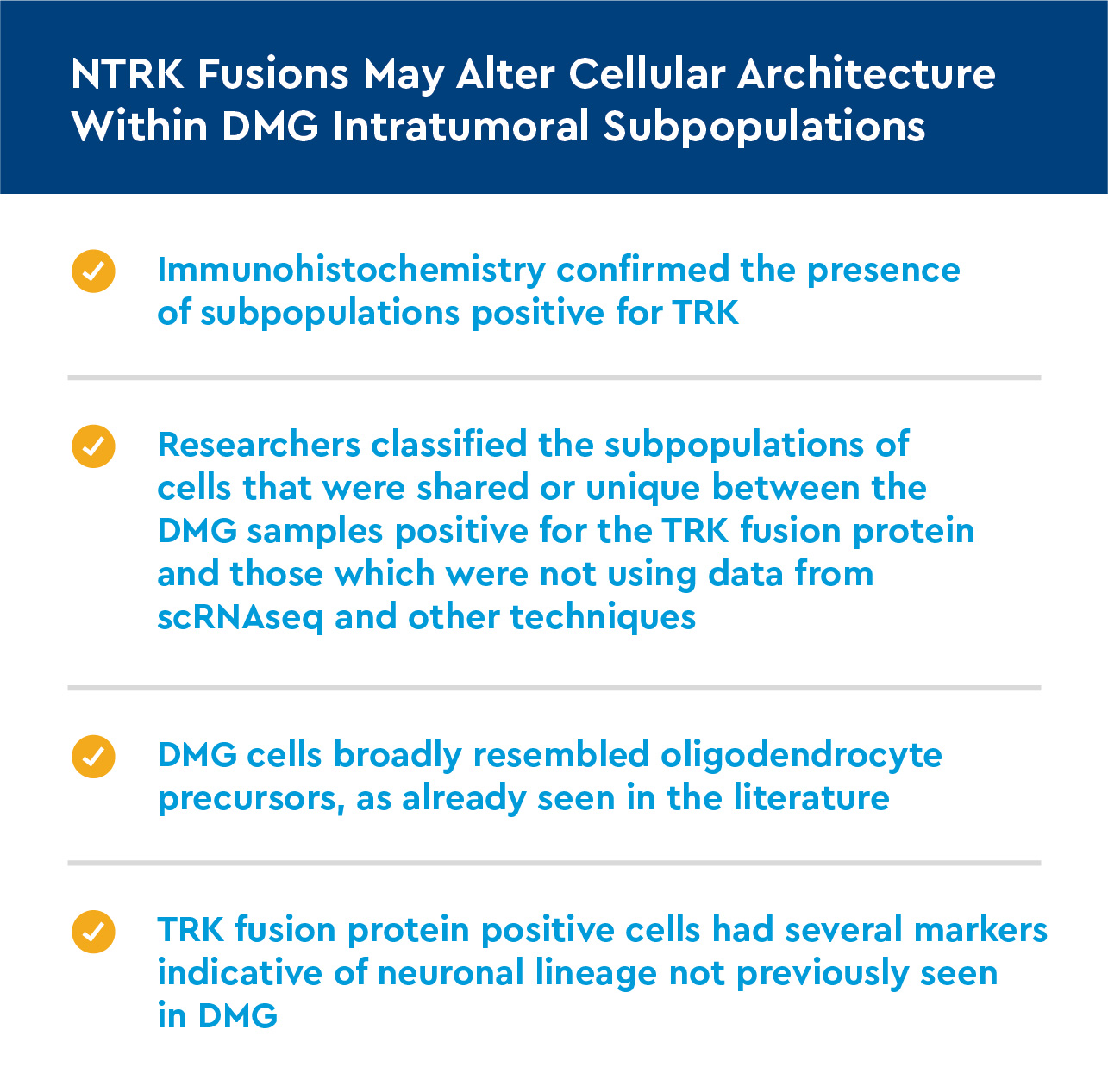
- Immunohistochemistry was performed on the samples so that microscopic characteristics could be analyzed.
- The DMG TRK-positive tumor cells were cultured from one patient, as were control DMG tumor cells not positive for the TRK fusion protein. Both were exposed to TRK inhibitors for 3 days; cell viability was assessed 2 days later.
Results: TRK positive DMG tumor cells show different histological, DNA expression and TRK inhibitor response
NTRK fusions are associated with neuronal transcriptional programs in DMG
- Using bulk RNA seq data from two DMG patients positive for the TRK fusion protein and three DMG patients negative for it, we analyzed differences in genetic expression.
- Gene analysis found TRK-positive patients had greater expression of genes related to neuronal differentiation, neuronal morphogenesis and synaptic signaling organization.
NTRK fusion status correlates with ex vivo response to TRK inhibitors
- When exposed to the TRK inhibitors, the tumor cell culture taken from the DMG patient positive for the TRK fusion protein showed a decrease in live cell numbers of 59% for larotrectinib and 47% for entrectinib.
- This effect was not seen if the cells were not exposed to the TRK inhibitors.
- This decrease was not present in tumor samples from DMG patients negative for the TRK fusion protein.
Discussion: TRK inhibitors should be considered as therapeutic options for diffuse intrinsic pontine glioma patients with NTRK fusion mutations
For other pediatric brain tumors, genetic tumor characterization has helped identify rare mutations with specific therapeutic targets, particularly for low-grade gliomas and medulloblastomas. Clinicians have historically been hesitant to perform biopsies in DMG patients due to safety concerns, so potentially treatable genetic aspects have remained unidentified. Experienced neurosurgeons such as those at Children’s Colorado can safely do these biopsies, however, and biopsy should be routinely offered to patients.
Study authors identified genetic differences between DMG cells positive for the TRK fusion protein and those negative for it. Importantly, they also found that in cell culture, NTRK fusion protein-positive DMG cells appeared to respond favorably to TRK inhibitors already approved by the FDA.
While TRK inhibitors in this patient population are not likely curative, they might lengthen survival time and improve quality of life. Given the lack of treatment options, they are a promising potential option for DMG patients with NTRK mutations.
Conclusion: Biopsy, routine genetic testing should be performed on patients with DMG
TRK inhibitors offer a potential treatment option for the small number of patients with DMG who have NTRK fusion mutations. Genomic assessments of these patients should be routine to identify and begin targeted treatment to provide any benefit for survival or quality of life.
Featured Researchers

Nathan Dahl, MD
Pediatric hematologist-oncologist
Center for Cancer and Blood Disorders
Children’s Hospital Colorado
Assistant professor
Pediatrics-Hematology/Oncology and Bone Marrow Transplantation
University of Colorado School of Medicine





 720-777-0123
720-777-0123