Key takeaways
-
Our Breathing Institute is an international leader in chILD research.
-
Nintedanib is used in adults with pulmonary fibrosis but is not licensed for pediatric use.
-
This Phase 3 randomized trial studied nintedanib for treating pulmonary fibrosis due to chILD.
-
Results of the landmark trial add to the evidence supporting nintedanib use in this population.
Childhood interstitial lung disease (chILD) is a group of rare pediatric lung diseases that often involve a combination of genetic factors, environmental exposures, and autoimmune dysregulation, although the exact cause is unknown in some cases. With no licensed medications to treat chILD, treatment is typically immunomodulation therapy, which lacks clinical evidence.
Some children with chILD will develop pulmonary fibrosis. In adults, pulmonary fibrosis (or lung fibrosis) is treated by the licensed medication nintedanib. It works by inhibiting tyrosine kinases and their effects on other aspects of the disease, such as reducing inflammation and scarring in the lungs. The drug has been shown to slow the decline in lung function in patients with idiopathic pulmonary fibrosis (IPF) and other forms of progressive pulmonary fibrosis. Given the efficacy of nintedanib in adults, InPedILD trial investigators believed it could provide a similar benefit in this pediatric population.
Robin Deterding, MD, chair of the international chILD Research Network and director of the Breathing Institute at Children's Hospital Colorado, was the corresponding investigator for this Phase III double-blind, randomized, placebo-controlled trial. Dr. Deterding leads the chILD Program at Children’s Colorado, one of the 43 sites of the 21-country trial.
Methods: pediatric patients with fibrosing chILD in nintedanib trial
Pediatric patients were enrolled in the trial based on the following inclusion criteria:
- Ages 6 to 17 years old
- Fibrosing ILD on a high-resolution computed tomography (HRCT) scan
- Clinically significant disease
- ≥3 Fan score, or
- Documented evidence of clinical progression
Trial design
- Participants screened for four weeks, randomized with a 2:1 ratio, and stratified by age group
- 6 to 11 years old
- 12 to 17 years old
- Received nintedanib or a placebo for 24 weeks; then all received open-label nintedanib for a variable period
- Dosing based on weight-dependent allometric scaling; dose reductions and treatment interruptions permitted to manage adverse events
Recruitment closed after ≥30 patients completed pharmacokinetic sampling at week 26, or prematurely discontinued the trial. All patients were scheduled for an end-of-treatment visit, then either began a 4-week follow-up or rolled over into open-label extension trial, with all receiving nintedanib.
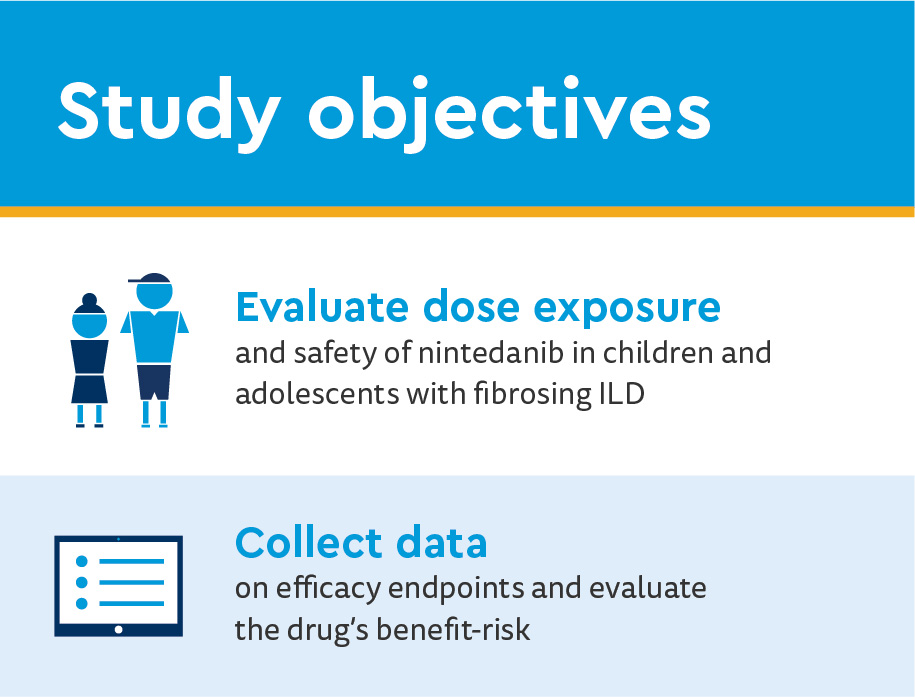
Co-primary endpoints
- Area under the plasma concentration–time curve at steady state at week 2 of nintedanib
- Proportion of patients with treatment-emergent adverse events at week 24
Safety endpoints
- Adverse events (AE)
- Coded according to the Medical Dictionary for Regulatory Activities
- Pathological findings on bone imaging, stunted growth of the dental root on dental imaging considered AE of special interest
- Change from baseline height-for-age z-score at week 24
Efficacy endpoints
- Changes in FVC percentage predicted
- Oxygen saturation (SpO2) at rest
- 6-minute walk test distance
- Week 24 Pediatric Quality of Life™ scores
- Self-reports by age range
- Parent report
Results: findings from a randomized trial of nintedanib in pediatric patients
Nintedanib treatment group characteristics
- 8 in 6 to <12-year age group
- 18 in 12 to <18-year age group
- 147.0 mean height, cm
- 40.9 mean weight, kg
- Top 3 diagnoses
- 10% other
- 7% surfactant protein deficiency
- 4% systemic sclerosis
- 1,633 mean FVC, ml
Placebo treatment group characteristics
- 4 in 6 to <12-year age group
- 9 in 12 to <18-year age group
- 148.4 mean height, cm
- 44.7 mean weight, kg
- Top 3 diagnoses
- 5 percent surfactant protein deficiency
- 4 percent other
- 3 percent systemic sclerosis
- 1,932 mean FVC, ml
Nintedanib group exposure
- 22.3 weeks mean (double-blind period)
- 46.5 weeks mean (entire trial)
Placebo group exposure
- 22.6 weeks mean (double-blind period)
- 49.6 mean (entire trial; placebo then open-label nintedanib)
Pharmacokinetics
Geometric mean for nintedanib (similar to the geometric means from adult studies)
- 175 ug*h/L (6 to11-year-olds)
- 160 ug*h/L (12 to 17-year-olds)
Entire trial
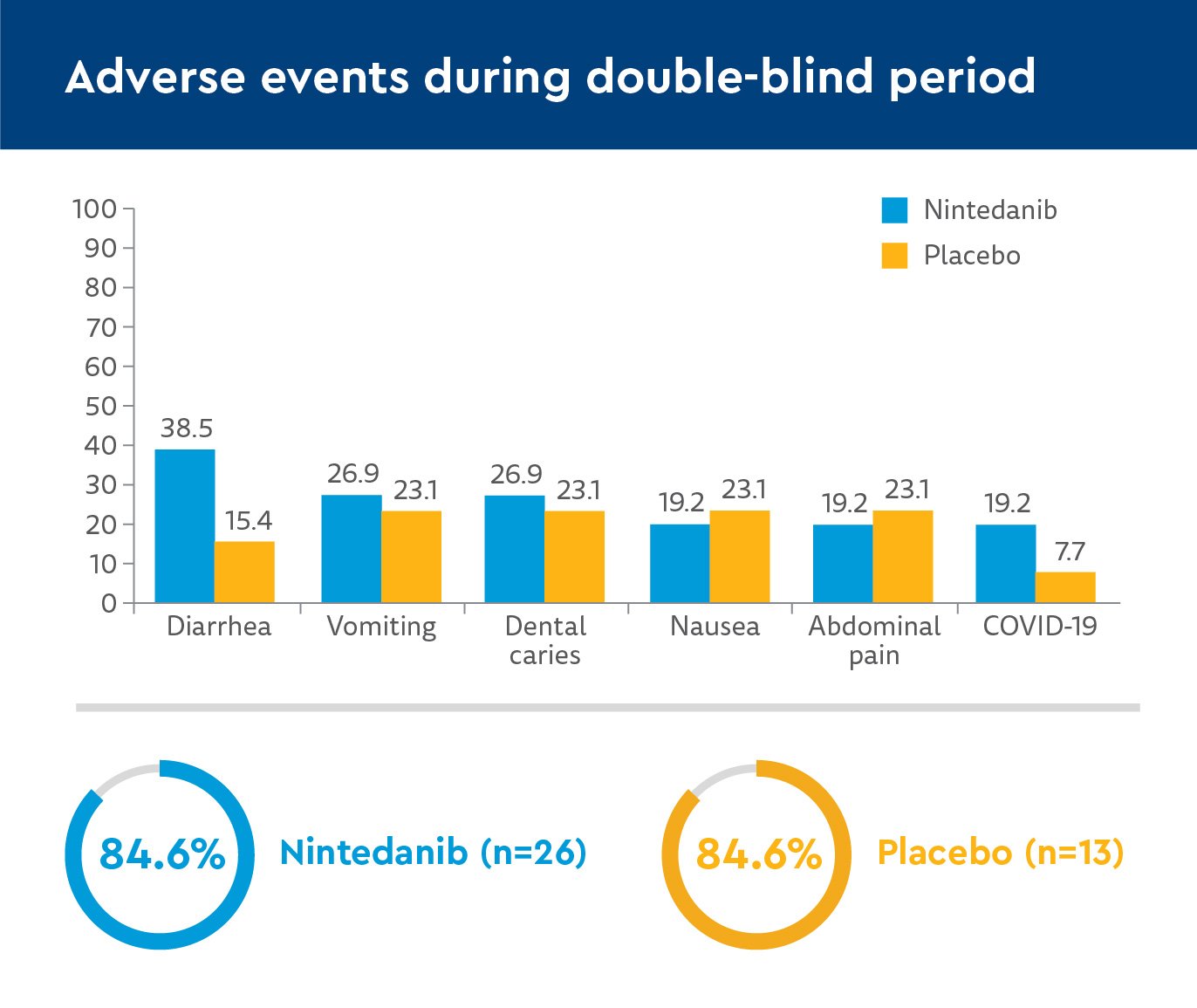
- 100% experienced adverse events in nintedanib group
- 19% reported serious events
- 92.3% experienced adverse events in placebo group, then open-label nintedanib
- 23.1% reported serious events
Adjusted mean changes in FVC predicted at week 24
- 0.3% nintedanib group
- Treatment effect within range observed in comparable adult trials
- −0.9% placebo group
InPedILD trial key findings
- The InPedILD trial is the first international placebo-controlled trial conducted for lung fibrosis due to chILD.
- Nintedanib demonstrated acceptable safety profile in this pediatric population.
- Diarrhea was the most common adverse event, as in adult trials, but no patients discontinued participation because of it.
- A weight-based dosing regimen achieved exposure in pediatric patients that is comparable to adult trials.
- Changes in lung function and oxygen saturation during the trial suggest potential benefits of nintedanib, but more data is needed.
Conclusion: more evidence supporting nintedanib for lung fibrosis due to chILD
This landmark study is an important step forward in understanding and treating this condition. Combined with what is known about the effects of nintedanib in adults with lung fibrosis, the results of the InPedILD trial provide further evidence to support the use of nintedanib in children and adolescents with lung fibrosis due to chILD.
Featured Researchers

Robin Deterding, MD
Chief, Pediatric Pulmonary Medicine
The Breathing Institute
Children's Hospital Colorado
Professor
Pediatrics-Pulmonary Medicine
University of Colorado School of Medicine





 720-777-0123
720-777-0123