Key takeaways
-
This mouse model research found a difference in liver B-cell composition, with a transition to a more mature and activated innate immune cell phenotype.
-
Activation of T-cells and macrophages by B-cells occurs independent of antigen presentation.
-
B-cells in the biliary atresia liver are most likely participating in the inflammatory process through increased production of pro-inflammatory cytokines.
Research background: B-cells have been ignored for far too long
Biliary atresia (BA) is a pediatric liver disorder caused by inflammation and scarring of the bile ducts. In BA, the blocked biliary tree damages the liver, necessitating the need for an eventual liver transplant.
The disease is believed to be a progressive inflammatory process, and as such, the role of immune cells is widely studied. However, most studies look at the role of T-cells in BA, whereas the role of B-cells (as activators of T-cells and macrophages) have drawn little attention, until now.
Children’s Hospital Colorado completed a series of studies using mouse models to investigate the role B-cells have in the activation of T-cells during the course of BA.
In biliary atresia, are T-cells activated through B-cell antigen presentation or B-cell cytokine production?
This team previously reported on a mouse strain (Ig-a-/-) that has no B-cells and does not develop the mouse form of BA. Thus, there is strong evidence to suggest a prominent role for B-cells in BA.
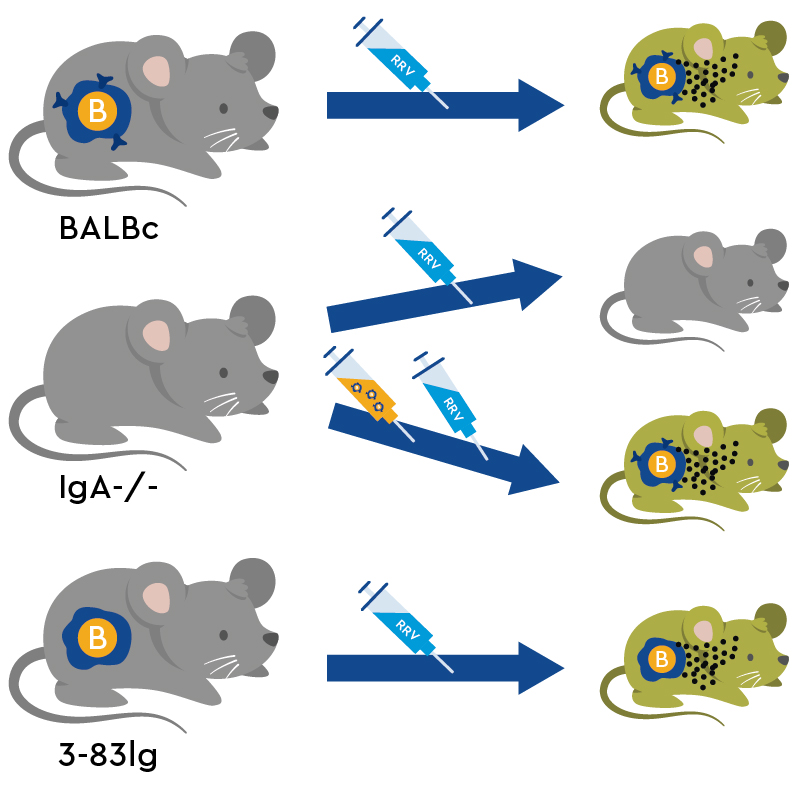
Next, they wanted to determine the B-cell mechanisms associated with biliary disease, specifically the role of B-cells in activating T-cells through presenting antigens and/or producing stimulating cytokines.
Research methods part I: mice with and without B-cells
A standard strain of mice, BALB/c, develops a liver disorder very similar to human BA when injected with the Rhesus rotavirus (RRV). The B-cells in these RRV-infected mice (14-day-old “BA-modeling” mice), are elevated overall. Additionally, there is a shift in the proportions from immature to mature and activated B-cells. Control mice, injected with a balanced salt solution, have fewer B-cells which were characterized as immature.
Using the BA-modeling mice and the BA-resistant (Ig-a-/-) mice, researchers tried to induce BA in the BA-resistant mice using adoptive transfer. Here, B-cells from control mice and from BA-modeling mice were injected into BA-resistant mouse mice (24 hours old). They were then injected with RRV or saline control. Lastly, they included a group of mice with B-cells that express inflammatory cytokines but do not engage in the antigen presentation (3-83Ig knock-in mice) that activates T-cells.
Researchers used this new strain to determine if they can develop the murine form of BA without the ability to present antigen and only the ability to produce cytokines.
Research results: Biliary atresia-resistant mice can develop BA symptoms through a transfer of B-cells
When BA-resistant mice received an adoptive transfer of B-cells from either control or BA-modeling mice and were then infected with RRV, there was a significant increase in T-cells and macrophages in the liver, reminiscent of BA. By examining liver tissues, researchers found inflammation around the bile ducts, noticeable dead cells and an increase in the number of bile ductuli (evidence of bile duct obstruction). With the transfer of B-cells, the Ig-a-/- mice succumbed to RRV infection and developed characteristics of BA. Lastly, the RRV infection of 3-83Ig knock-in mouse also resulted in characteristics of BA, suggesting the role of B-cells in BA is not through antigen presentation to the T-cells.
Research methods part II: the key to revealing how B-cells operate in biliary atresia
Based on this evidence, T-cells were co-cultured with B-cells to determine if the elevated expression of inflammatory markers was sufficient for activating the T-cells. In this study, T-cells were plated onto a culture dish and B-cells were cultured in a well above the T-cells. A permeable membrane physically separated the two cell types but would allow the passing of soluble components like cytokines.
Research results: Cytokine expression from B-cells potentiates T-cell activation
B-cells from saline control mouse livers failed to activate T-cells in the co-culturing experiment, but BA-modeling liver B-cells induced a significant activation in T-cells as measured by the expression of CD69.
Activation of T-cells, in this manner and by these cells, strongly supports the theory that B-cells initiate the immune response in BA through cytokine activation of other immune cells.
Research discussion: B-cells contribute to biliary atresia through antigen-independent pathways
This study supports the notion that liver B-cells have a prominent, if not necessary, function in the progression of BA. There are elevated numbers of B-cells in the liver of children with BA, and the mouse studies suggest that they might have different characteristics than B-cells found in children who do not have BA.
By using different types of mouse models, Dr. Mack and her team found that BA could be induced in a mouse that is normally resistant to BA by transferring B-cells from a mouse strain that is susceptible to BA. Their final studies suggest that during the course of BA, B-cells work through the production of cytokines rather than as antigen presenting immune cells.
Although a young mouse modeling BA is different than a human child with BA, these studies lay the foundation for additional clinical studies in the field.





 720-777-0123
720-777-0123










