Between operations, pediatric orthopedic surgeon Nancy Hadley-Miller, MD, sips tea and looks at X-rays. She pulls the femur and tibia of an 8-year-old girl. On the righthand head of the tibia, just below the knee joint, the clean black line of the growth plate breaks up into a smear of grey, marbled and indistinct. The fracture punched through the growth plate, and when it healed it healed as bone. Effectively sealed, the plate can't expand. The bone can't grow.
"Trampoline injury, if I'm not mistaken," she remarks.
In her 30-plus years as an orthopedic surgeon, Dr. Hadley-Miller has seen thousands of injuries like these. At Children's Hospital Colorado alone, the Orthopedics Institute sees 150 to 160 fractures a week, about 30% of which affect the growth plate. Of those, 3% might form a "bony bar" like that of Dr. Hadley-Miller's 8-year-old patient. On average, that's perhaps one a week.
"These cells are communicating, like a little city. There's a whole circle of life in there we know almost nothing about."
- NANCY HADLEY-MILLER, MD
If the bar fuses less than half the growth plate, Dr. Hadley-Miller and surgeons like her can resect it and fill the area with a material to stop it from reforming: sometimes a fat graft, sometimes bone cement. Neither option is great. Fat degrades and melts away. Bone cement goes on hot, inflaming the surrounding tissue, and while it lasts longer, it can't grow with the patient.
That's best-case scenario.
When growth plates get injured
In many more cases, surgeons injure the counterpart limb — killing its growth plate, too. Given her age, that was the only option for the 8-year-old on Dr. Hadley-Miller's screen. Her tibia won't ever grow, but at least she won't have an angular deformity.
"There's a real need for better treatment options," says Karin Payne, PhD, Dr. Hadley-Miller's research partner and Director of the Payne Regenerative Orthopedics Lab at the University of Colorado School of Medicine, which shares both campus and faculty with Children's Colorado. "Currently there's no clinical treatment that can regenerate growth plate tissue."
Unlike bone, the growth plate comprises five distinct zones. Cartilage cells form in the top zone, proliferate in the next and hypertrophy after that. Then they die and calcify, to be broken down by osteoblasts and, finally, replaced by mineralized bone tissue. This process continues through skeletal maturity, when the plates fuse.
Engineering the environment for growth plate regeneration
The problem has to do with the mechanism of healing. Inflammatory cells invade the area and lay down the fibrous tissue necessary to stitch it back together, along, some studies have suggested, with an influx of mesenchymal stem cells. In the second week, blood vessels begin to form, which cues the stem cells to become bone.
"But if you can create an environment that promotes cartilage," says Dr. Payne, "it's possible to make those stem cells become cartilage instead."
Her lab is currently testing that idea on animal models of bony bars using a number of delivery mechanisms, in collaboration with bioengineers, biochemists and computing experts from all over the state. They're testing factors and combinations of factors that block angiogenesis, that attract stem cells, that mimic the chemical conditions under which cartilage develops and grows.
Cartilage regeneration that gels
Working with biopolymer expert Melissa Krebs, PhD, of the Colorado School of Mines, Dr. Payne's team is testing microgels made of chitosan, a biomaterial derived from crustacean cells. They're using them as a delivery mechanism for antibodies that block blood vessel formation, as well as factors that attract stem cells from surrounding tissue to encourage cartilage growth. Those studies are separate for now, but the aim of the National Institutes of Health grant that funds them is to bring them together.
Meanwhile, in a collaboration with materials science expert Stephanie Bryant, PhD, of the University of Colorado Boulder, the team is testing a biomimetic hydrogel delivery system based on polyethylene glycol, a synthetic biomaterial ideal for attaching biofactors. To this one they attach factors that promote cartilage growth and the environment it likes to grow in.
"Once we put mesenchymal stem cells into this hydrogel," says Dr. Payne, "they start producing collagen type 2 and sulfated glycosaminoglycans" — the chemical backbone, so to speak, of cartilage.
The mechanical properties of the growth plate
These projects show promise, but the project Dr. Payne is most excited about — the one she thinks is closest to clinical practice, maybe even just a few years out — is more tangible still. Conceived to replace the fat grafts or bone cement currently used to prevent bony bars, it's a personalized, 3D printed implant that blocks bony bar reformation and stabilizes the growth plate, ideally while the tissue regenerates.
For that, her lab collaborates with Dr. Bryant and CU Boulder bone mechanics expert Virginia Ferguson, PhD. Together, they're working to match the mechanical properties of the implant to each of the growth plate's five zones — an aspect of the growth plate that, so far, no research team has ever tried to deduce.
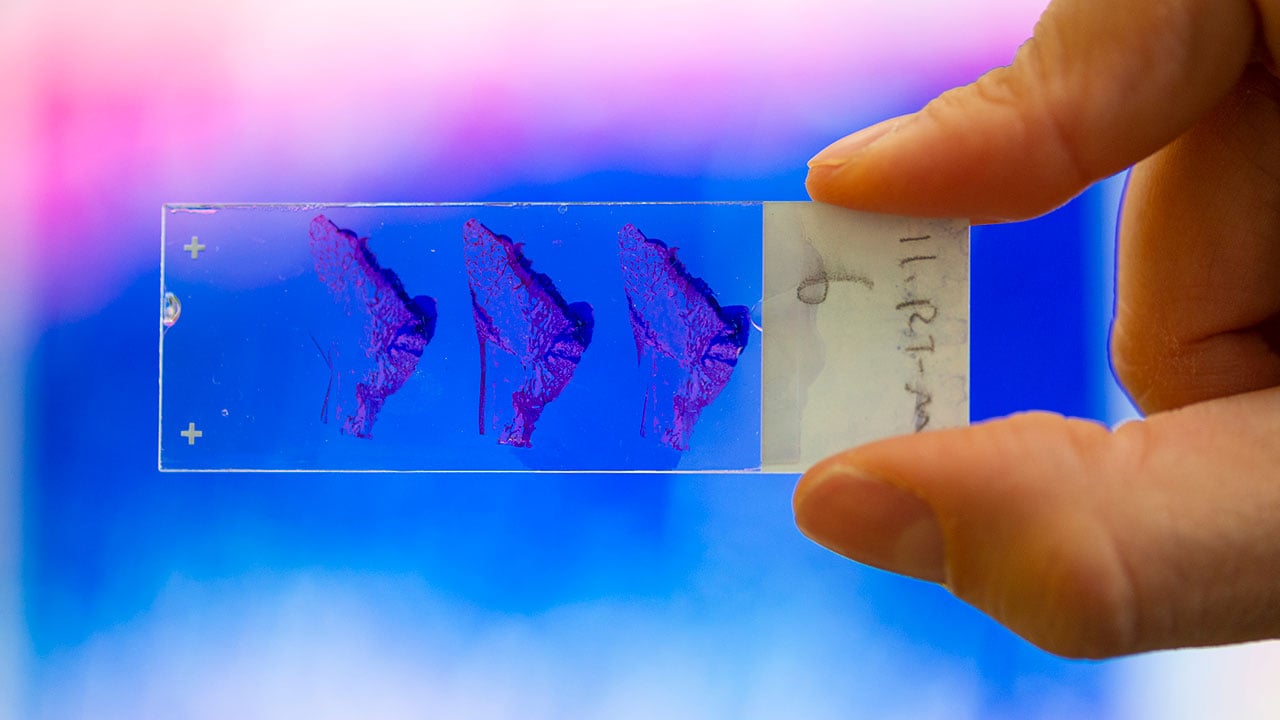
Fabricated as an interlocking system of pillars, the implants can then be infilled with cartilage-promoting hydrogels. In the lab, a cadre of grad students, research assistants and fellows resect tibia and humeri the length of thumbtacks, dry them, pack them in paraffin and chop them five microns thick. Stained, the slides provide the proof: bright blue eruptions of cartilage where, compared to samples left untreated, the bar reformed.
"It's fascinating," says Dr. Hadley-Miller, who contributes both bench and clinical work to the project. "What keeps these cells from spreading out instead of sticking together? How do the cells progress downward? What are the signals that turn them on and off? They're communicating, like a little city. There's a whole circle of life in there we know almost nothing about."
Mapping the outcomes of growth plate injury
The clinical picture of growth plate injury isn't much clearer. On that side, Dr. Hadley-Miller and her team at Children's Colorado's Musculoskeletal Research Center, or MRC, are assembling a comprehensive study — another first for the field.
Epidemiologist Patrick Carry, MS, can get a good idea of the incidence of fractures that affect the growth plate by pulling from a number of national trauma databases. But what those databases can't offer is longitude.
"As a Level 1 Trauma Center, we get a lot of kids referred with existing growth plate injuries, but we don't know how many started with a fracture that developed into a growth plate injury, or how many of those injuries resulted in these growth plate disturbances," he says. "So we're developing a strategy to answer that question."
As one of the first pediatric institutions in the U.S. to adopt an electronic medical record system, Children's Colorado has comprehensive data stretching back well more than a decade. The question is how to access and sort it.
Carry, an expert in study design, is currently working out the inclusion criteria with a targeted chart review of bones and trauma types that tend toward growth plate injury. When the charts are pulled, he and his team will comb through thousands of X-rays and diagnostic codes, a huge, time-intensive effort requiring many, many man hours.
Getting the pieces into place
That kind of effort is largely possible through the unique funding model of the MRC, which pools clinical dollars earned from surgery toward an infrastructure of 14 research assistants and two statisticians who power dozens of surgeon-directed research efforts. As the MRC's first such research assistant, Carry has been working with Dr. Hadley-Miller, the MRC's medical director, for more than ten years.
But their growth plate work started with a joint research grant opportunity between Children's Colorado and the School of Mines. At the time, Dr. Payne was working in adult cartilage regeneration, mostly focused on degenerative joint disease. She hadn't done growth plate research before. Neither, for that matter, had Dr. Hadley-Miller. Her 30-year career has been focused on the molecular genetics of scoliosis.
She heard about the grant talking to a colleague after a surgery, and she ran it past Dr. Payne, whose office was just a few doors down. As it turned out, Dr. Payne knew someone they could work with at Mines. For her, it represented a new and interesting research direction. For Dr. Hadley-Miller, it was something like a return.
"In fellowship I always wanted to do something with growth plate," she recalls. "But I didn't find a bone lab to work in, didn't have the mentorship. That's how I ended up in scoliosis. I followed another idea. It wasn't a bad way to go."
Now, though, with funding from both the Gates Grubstake Fund and the NIH, their lab is just one of a few in the world doing groundbreaking work in growth plate regeneration, and it's leading the field. For Dr. Payne, that conversation has fundamentally altered the course of her career.
Some of that is coincidence, Dr. Hadley-Miller acknowledges. But more than that, it's having the pieces in the right place.
"Patient care is our bread and butter," she says. "Here, we're using it to pay for research. But you really have to want to do it. You have to invest in the people and the infrastructure to make those connections happen."
She knocks back the rest of her tea. And with that, she's off to surgery.
Featured Researchers

Nancy Hadley-Miller, MD
Orthopedic Surgery
Children’s Hospital Colorado
Professor
Orthopedics
University of Colorado School of Medicine
Karin Payne, PhD
Associate professor
Orthopedics
University of Colorado School of Medicine
Virginia Ferguson, PhD
Assistant professor
University of Colorado - Boulder
Patrick Carry, MS, PhD
Assistant professor
Orthopedics
University of Colorado School of Medicine





 720-777-0123
720-777-0123