Key takeaways
-
Treatment of MMC can lead to hysterectomy complications, such as uterine scarring.
-
A new technique used by our fetal surgeons resulted in a significantly lower rate of complications.
-
This may reduce obstetric morbidity associated with prenatal MMC repair and other open fetal surgical procedures.
Research background: Myelomeningocele repair can result in uterine scar complications
The Management of Myelomeningocele Study (MOMS) was a ground-breaking study of the treatment of myelomeningocele (MMC), the most severe form of spina bifida. The study, which compared fetal versus postnatal surgery to repair spina bifida, found that fetal surgery had many long-term benefits and some risks.
A follow-up to the MOMS study evaluated 88 uterine scars at the time of delivery. Abnormalities were found in 35% of the scars:
- 23.9% very thin scar
- 9.1% partial dehiscence (wound opened along incision)
- 2.3% complete dehiscence
The hysterotomy (incision in the uterus) complication rate is a significant limitation of prenatal repair of MMC by open fetal surgery and can also significantly affect future reproductive outcomes.
Due to this known complication, fetal surgeons at the Colorado Fetal Care Center at Children's Hospital Colorado modified their hysterotomy closure technique for MMC fetal surgery at the inception of their fetal surgery program. They reviewed their experience from 2013 to 2016 by assessing hysterotomy complication rates of their patients at the time of cesarean delivery.
Research methods: MMC fetal surgery patients were reviewed, surgical techniques compared
Patients who underwent open fetal repair between 2013 and 2016 were retrospectively reviewed. All patents underwent a comprehensive evaluation, including:
- Detailed ultrasound and Fetal MRI
- Level of fetal myelomeningocele defect
- Degree of hindbrain herniation
- Degree of ventriculomegaly
- Associated structural anomalies
- Amniocentesis
- Fetal echocardiogram
- Independent counseling about obstetric complications
They also had to pass a psychosocial evaluation and meet the MOMS trial criteria to be eligible. All approved patients were offered open fetal surgery between 23 0/7 weeks and 25 6/7 weeks gestation. Thirty-three patients delivered at their referring center and 10 patients delivered at the Colorado Fetal Care Center.
Surgical technique
Standard uterine closure following MMC repair:
Placement of a series of interrupted full-myometrial-thickness #0 polydioxanone retention sutures placed every 2 to 3 cm followed by a running #0 PDS suture to re-approximate the stapled myometrial edges. Once the myometrial edges are approximated, the interrupted retention sutures are tied.
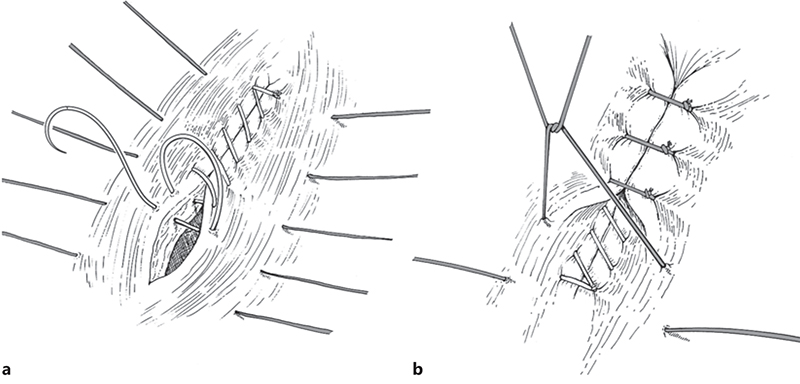
Colorado Fetal Care Center's modified technique:
A third imbricating layer performed by interrupted #0 PDS U-stitches resulting in serosal-to-serosal apposition superior to the underlying closure An omental patch is then placed over the hysterotomy closure as was used in the MOMS trial.

Research results: Significant reduction in complications overall vs. MOMS trial technique
During the study period, 49 patients met the requirements to be evaluated. Compared to the updated MOMS cohort, researchers found:
The addition of the third imbricating layer at the time of the hysterotomy closure resulted in a significantly lower than expected complication rate compared the MOMS trial technique.
Research conclusion: Obstetric morbidity may be reduced in prenatal MMC repair
This was the first report of open fetal surgical outcomes outside of the MOMS trial and its three study centers. It represents a post-MOMS realty of practicing outside the rigorously controlled study and allows patients to return to their referring providers for the remainder of their pregnancy.
The simple modified closure may reduce obstetric morbidity associated with prenatal MMC repair and other open fetal surgical procedures.
Contact us
The Colorado Fetal Care Center offers comprehensive fetal and maternal care for women facing complex or high-risk pregnancies. Contact us at CFCC@childrenscolorado.org or 720-777-4463.
The study was published in the Sept. 6, 2017, issue of Fetal Diagnosis Therapy.





 720-777-0123
720-777-0123










