- Doctors & Departments
-
Conditions & Advice
- Overview
- Conditions and Symptoms
- Symptom Checker
- Parent Resources
- The Connection Journey
- Calm A Crying Baby
- Sports Articles
- Dosage Tables
- Baby Guide
-
Your Visit
- Overview
- Prepare for Your Visit
- Your Overnight Stay
- Send a Cheer Card
- Family and Patient Resources
- Patient Cost Estimate
- Insurance and Financial Resources
- Online Bill Pay
- Medical Records
- Policies and Procedures
- We Ask Because We Care
Click to find the locations nearest youFind locations by region
See all locations -
Community
- Overview
- Addressing the Youth Mental Health Crisis
- Calendar of Events
- Child Health Advocacy
- Community Health
- Community Partners
- Corporate Relations
- Global Health
- Patient Advocacy
- Patient Stories
- Pediatric Affiliations
- Support Children’s Colorado
- Specialty Outreach Clinics
Your Support Matters
Upcoming Events
Colorado Hospitals Substance Exposed Newborn Quality Improvement Collaborative CHoSEN Conference (Hybrid)
Monday, April 29, 2024The CHoSEN Collaborative is an effort to increase consistency in...
-
Research & Innovation
- Overview
- Pediatric Clinical Trials
- Q: Pediatric Health Advances
- Discoveries and Milestones
- Training and Internships
- Academic Affiliation
- Investigator Resources
- Funding Opportunities
- Center For Innovation
- Support Our Research
- Research Areas

It starts with a Q:
For the latest cutting-edge research, innovative collaborations and remarkable discoveries in child health, read stories from across all our areas of study in Q: Advances and Answers in Pediatric Health.


Vaccination Deliberation: Learn About the COVID-19 Vaccines

COVID vaccine options and information
It's normal to have questions. We’ve tracked down the answers to common questions about the COVID-19 vaccines. Wherever you are on your journey, knowing the facts can help.
The top questions (and answers)
Open the windows below to read the answers to the most common questions we hear. If your question isn’t listed, you can view our complete list of vaccine FAQs or submit a question of your own.
While the vaccines to prevent COVID-19 are newer vaccines, we actually know quite a lot about them. Also, after more than a year of widespread immunization, we have a lot of information and experience proving the safety of the currently authorized COVID-19 vaccines.
Here are the top facts to know about the COVID-19 vaccines that have been recommended for use in the U.S.
- The COVID-19 vaccines are safe and effective.
- Getting sick with the virus that causes COVID-19 is much more likely to cause you long-term problems than the vaccine.
- You cannot get COVID-19 from the vaccine.
- You may have temporary side effects after vaccination. These are normal and should go away in a few days.
- The vaccines remain highly effective at preventing severe COVID infections that can lead to hospitalization and death.
- Hospitalizations and deaths from COVID-19 are much more common in people who have not been vaccinated.
Medical experts are still studying several longer-term questions about the COVID-19 vaccines. We’ll learn even more about the vaccines as additional studies are published.
Here are a couple of things we don’t know – yet.
- We’re still learning how long COVID-19 vaccines, including boosters, protect people.
- We are still learning how well each vaccine works against new variants of the coronavirus that causes COVID-19. So far, current COVID-19 vaccines are very effective at preventing severe infections, hospitalizations and deaths – including newer variants.
Most children who contract COVID-19 don't get seriously ill or need hospitalization. However, some children do get very sick from the virus, even those who have no preexisting conditions.
While severe illness, hospitalization and death from COVID-19 is rare in children, some kids may experience lingering symptoms (Long COVID) or be at risk for a rare but serious inflammatory condition that can affect the heart (multisystem inflammatory syndrome, or MIS-C). Getting the COVID-19 vaccine is the best way to protect people of all ages from becoming severely ill or having long-lasting health impacts due to the virus.
Research has shown that getting your child the vaccine:
- Lowers their risk of getting infected with COVID-19
- Lowers their risk of getting MIS-C
- Keeps them from getting seriously sick if they do get COVID-19
- Keeps them from spreading COVID-19 to others, including family members who are at high risk of getting very sick or dying from the virus
Medical and public health experts, including at the CDC, the American Academy of Pediatrics and Children’s Hospital Colorado, recommend that all eligible children and adolescents get the COVID-19 vaccine to help protect them from contracting and spreading the virus.
In June 2022, the Food and Drug Administration and CDC expanded the age groups for both Pfizer's and Moderna's COVID-19 vaccines. Both vaccines are now available for children as young as 6 months.
Both the Pfizer and Moderna vaccines were proven to be safe and effective in children 6 months and older. Both vaccines use mRNA technology, but there are differences in the number of doses and schedules.
COVID-19 vaccine doses and schedules for children under 5
Pfizer
- Pfizer's vaccine for kids younger than 5 is one-tenth (1/10) of the dose for people 12 and older, or 3 micrograms.
- 3 shots are needed: the first 2 are given 3 weeks apart and the last at least 2 months later.
Moderna
- Moderna’s vaccine for kids under 5 is one-quarter (¼) of the adult dose, or 25 micrograms.
- 2 shots are needed, approximately 4 weeks apart.
Doctors recommend choosing whichever vaccine is easiest to obtain for your child.
Pregnant people have a higher risk of getting COVID-19 and are at higher risk of severe COVID-19 disease. We now know that getting sick with COVID-19 during pregnancy is associated with increased risk of pregnancy complications, including preterm delivery (premature birth for the baby). Getting the COVID-19 vaccine during pregnancy helps protect you from getting very sick with COVID-19.
The American Academy of Obstetricians and Gynecologists and CDC strongly recommend that pregnant patients get the COVID-19 vaccine. If you are pregnant and have questions about the COVID-19 vaccines, talk to your OBGYN.
In the video below, pediatrician Lisa DeCamp, MD, answers this question.
Hear what our experts have to say
You submitted your questions, and we found answers. Watch videos featuring our pediatric experts answering common questions about the COVID-19 vaccines and children.
Click around the playlist to get answers to the questions that matter most to you.
Related reading
The truth about vaccines: what parents need to know
Learn about vaccines and how they work to provide the best possible protection against harmful diseases in children.
Children and COVID-19: debunking the number one myth
You might have heard that children aren’t affected by COVID-19. But is that true? We asked our experts to explain all the ways that the pandemic has impacted children’s health – and what we can do about it. Learn more.
Coronavirus disease 2019 (COVID-19) in children
Learn about signs, symptoms, diagnosis and treatment of the coronavirus in children. See how our specialists treat and diagnose pediatric COVID-19 cases.
Where to go, when you're ready
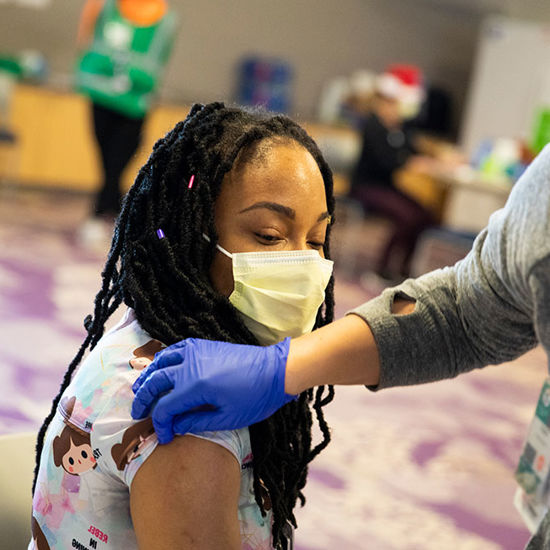
Ready to get vaccinated?
Vaccines are now more readily available, and there are resources available to help you find a vaccine appointment. When you’re ready, here’s how to find – and get to – a vaccine clinic near you.
What’s your reason?
We asked community members why they decided to get vaccinated against COVID-19. Click through the photos to read their answers.
Share your why
Did these resources help you? Please consider sharing this information with the people you love.



 720-777-0123
720-777-0123