Key takeaways
-
Group 3 medulloblastoma patients face poor prognosis and lack effective treatment options.
-
The oncogenic kinase PLK1 controls cell cycle and growth and is a cancer therapeutic target.
-
Our researchers found that onvansertib, a novel orally available PLK1 inhibitor, when combined with radiation, shows therapeutic promise.
-
A clinical trial to combine onvansertib with radiation in patients with Group 3 medulloblastoma is being developed.
Background: Can PLK1 inhibitor onvansertib be effective in Group 3 medulloblastoma patients?
Medulloblastoma, the most common malignant pediatric brain tumor, is a diverse and heterogenous disease with four major subgroups. The worst prognosis is in Group 3 patients, who often have high MYC expression, a transcription factor that triggers or blocks any array of target genes. Group 3 also lacks novel treatment options.
Polo-like kinase-1 (PLK1) is highly expressed in rapidly dividing normal cells and is overexpressed in many types of cancer, including Group 3 medulloblastoma, and is associated with poor prognosis. In pediatric solid tumors and brain tumors, PLK1 inhibition leads to apoptosis in vitro and in tumor xenografts. While PLK1 inhibitors have been used intravenously as a singular treatment or in combination with chemotherapy in a variety of cancers, most clinical trials were discontinued due to long-term adverse effects of treatment.
The novel PLK1 inhibitor, onvansertib, is the first available for oral administration and has shown tumor growth inhibition in hematologic tumors, osteosarcoma, ovarian carcinoma, breast cancer and colon adenocarcinoma cells. Current clinical trials using onvansertib include:
- Acute myeloid leukemia
- Prostate cancer
- Colorectal cancer
In this pre-clinical study led by Rajeev Vibhakar, MD, investigators from multiple departments at Children’s Hospital Colorado, including the Morgan Adams Foundation Pediatric Brain Tumor Research Program and the Neuro-Oncology Program for Children with Central Nervous System Tumors, sought to determine the effects of onvansertib on MYC-driven medulloblastoma as a monotherapy or in combination with radiation.
Methods: Identifying essential genes for medulloblastoma tumor growth
- Investigators used CRISPR-Cas9 screening to determine which genes were essential for medulloblastoma tumor growth.
- PLK1 expression was examined by performing microarray and immunohistochemistry on pediatric patient samples.
- In vitro effects of onvansertib measured by:
- Cell viability
- Colony-forming assays
- Extreme limiting dilution assay
- RNA-Seq
- Flow cytometry analyzed:
- ALDH activity
- Cell-cycle distribution
- Apoptosis
- Immunofluorescence staining assessed DNA damage.
- Monotherapy, or radio-sensitizing effect, was explored through medulloblastoma xenograft studies.
Results: PLK1 is a critical mediator in MYC-driven medulloblastoma
CRISPR-Cas9 screen

This included:
- Cell cycle and DNA replication are top signaling hubs for medulloblastoma cell growth.
- Multiple genes negatively impact overall cellular fitness and are targets for effective treatment.
- PLK1 is a top negatively selected gene.
An investigation of PLK1 expression in DepMap found PLK1 is a critical mediator in 55 central nervous system cell lines, including eight medulloblastoma cell lines.
Microarray-based gene expression analysis was performed on:
- 11 Group 3 medulloblastoma patients
- 6 normal cerebella samples
- 763 recently described medulloblastoma samples
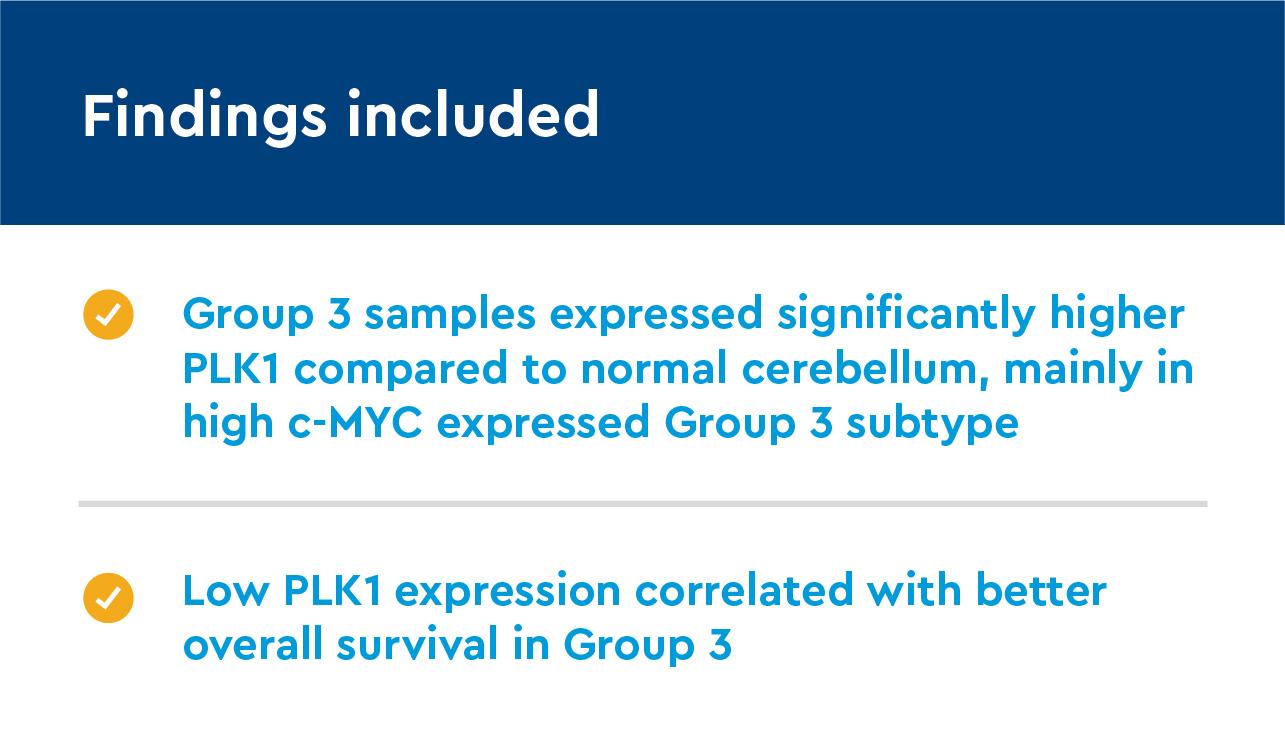
An examination of PLK1 and Ki67 expression in medulloblastoma subtypes demonstrated high expression of PLK1 is associated with high malignancy and poor prognosis in Group 3.
PLK1 inhibition with onvansertib suppresses tumor growth in Group 3 medulloblastoma
- Markedly inhibited survival of medulloblastoma cell lines with varying potency
- IC50 concentrations of onvansertib in Group 3 medulloblastoma cell lines were in a low nanomolar range.
- Significant time- and dose-dependent sensitivity, suggesting PLK1 function is essential for medulloblastoma cell survival
- Decreased levels of p-CDC25C, indicating inhibition of PLK1 activity
- Dramatically suppressed adhesion-independent colony formation and tumor cell growth in Group 3 medulloblastoma cells
- Suppressed neurosphere growth
- Reduced capacity for self-renewal of cancer stem cells, efficacy of neurosphere formation
Onvansertib induces G2/M arrest and suppresses proliferation in Group 3 medulloblastoma
- Large overlap of differential expressed genes between D425 and D458
- Significant enhancement in both cell lines’ G2M checkpoint, mitotic spindle, MYC targets, P53 pathway, and apoptosis gene sets
- Declined enrichment score of cell cycle checkpoints and transition gene sets
- Induced G2/M arrest of cell cycle
- Significant decrease in cyclin E protein, increase in cyclin B protein
- Reduced the protein level of CDK1 in medulloblastoma cells
- Attenuated expression signatures related to DNA replication, synthesis
Onvansertib suppresses MYC expression and transcriptional Activity
- Substantially downregulated MYC target genes in medulloblastoma cells
- Notable reduction of c-MYC protein abundance in medulloblastoma cells
- Affected ~50% differential expressed genes regulated by shRNA-mediated PLK1 depletion
- 6 of 9 hallmark gene sets in PLK1 depletion cells altered, indicating inhibition of PLK1 activity
- PLK1 knockdown suppressed proliferation of medulloblastoma cells, led to similar marked reductions in PLK1 and c-MYC mRNA and protein levels
Onvansertib increases apoptosis and attenuates tumor growth in vivo and in vitro
- Altered expression of apoptosis gene set
- Significantly increased caspase Group 3 activity, elevated level of cleaved PARP
- Markedly enhanced annexin V-positive population, indicating increased total percentage of apoptosis
- Reduced viable cells in two patient-derived short-term cultures from Group 3 medulloblastoma patients, demonstrating in vitro efficacy
- Strongly attenuated MB tumor growth in an orthotopic mouse model
- Treated cohorts survived markedly longer
- Dramatically improved the median survival of the PDX model
- Substantially depleted c-MYC expression concomitant with more intense expression of FBXW7
Onvansertib enhances radiation sensitivity of medulloblastoma
- Radiation built up P-γH2AX, drastically enhanced by onvansertib in both cell lines.
- Radiation elevated P-γH2AX expression, as observed in vivo.
- Combination treatment reduced colony formation compared with radiation alone.
- Radiation notably increased active caspase-3 activity.
- Combination treatment markedly amplified active caspase-3 activity compared with radiation alone.
- In vivo, tumor size visibly reduced within a week post-radiation.
- Combination treatment led to sustained tumor regression in D458 xenograft model.
- Combination treatment delayed tumor recurrence and improved overall survival compared to radiation treatment alone, as observed in MRI.
- Onvansertib can extend latency of tumor growth beyond treatment with radiation alone and may be effective treatment for combination therapy.
Discussion and conclusion: Onvansertib significantly boosts radiation impact in vitro and animal studies, clinical trial warranted

No preclinical study or clinical trial of onvansertib has yet been performed in any pediatric cancer. This is the first report to evaluate the therapeutic potential of onvansertib in medulloblastoma.
Study authors demonstrate the therapeutic potential of a novel orally available PLK1 inhibitor, onvansertib, in Group 3 medulloblastoma. Onvansertib significantly enhances the impact of radiation in vitro and in animal studies. These results justify developing a clinical trial to determine the safety and efficacy of combining onvansertib with radiation in patients with MYC-driven medulloblastoma. At the time of publication, study authors were developing a first pediatrics clinical trial for onvansertib targeting pediatric tumors.
Cardiff Oncology, makers of onvansertib, donated the drug for research use at Children’s Colorado and will fund the hospital’s upcoming clinical trial.
Featured Researchers

Rajeev Vibhakar, MD, PhD, MPH/MSPH
Pediatric oncologist
Center for Cancer and Blood Disorders
Children's Hospital Colorado
Professor
Pediatrics - Hematology/Oncology and Bone Marrow Transplantation
University of Colorado School of Medicine





 720-777-0123
720-777-0123