- Doctors & Departments
-
Conditions & Advice
- Overview
- Conditions and Symptoms
- Symptom Checker
- Parent Resources
- The Connection Journey
- Calm A Crying Baby
- Sports Articles
- Dosage Tables
- Baby Guide
-
Your Visit
- Overview
- Prepare for Your Visit
- Your Overnight Stay
- Send a Cheer Card
- Family and Patient Resources
- Patient Cost Estimate
- Insurance and Financial Resources
- Online Bill Pay
- Medical Records
- Policies and Procedures
- We Ask Because We Care
Click to find the locations nearest youFind locations by region
See all locations -
Community
- Overview
- Addressing the Youth Mental Health Crisis
- Calendar of Events
- Child Health Advocacy
- Community Health
- Community Partners
- Corporate Relations
- Global Health
- Patient Advocacy
- Patient Stories
- Pediatric Affiliations
- Support Children’s Colorado
- Specialty Outreach Clinics
Your Support Matters
Upcoming Events
Colorado Hospitals Substance Exposed Newborn Quality Improvement Collaborative CHoSEN Conference (Hybrid)
Monday, April 29, 2024The CHoSEN Collaborative is an effort to increase consistency in...
-
Research & Innovation
- Overview
- Pediatric Clinical Trials
- Q: Pediatric Health Advances
- Discoveries and Milestones
- Training and Internships
- Academic Affiliation
- Investigator Resources
- Funding Opportunities
- Center For Innovation
- Support Our Research
- Research Areas

It starts with a Q:
For the latest cutting-edge research, innovative collaborations and remarkable discoveries in child health, read stories from across all our areas of study in Q: Advances and Answers in Pediatric Health.


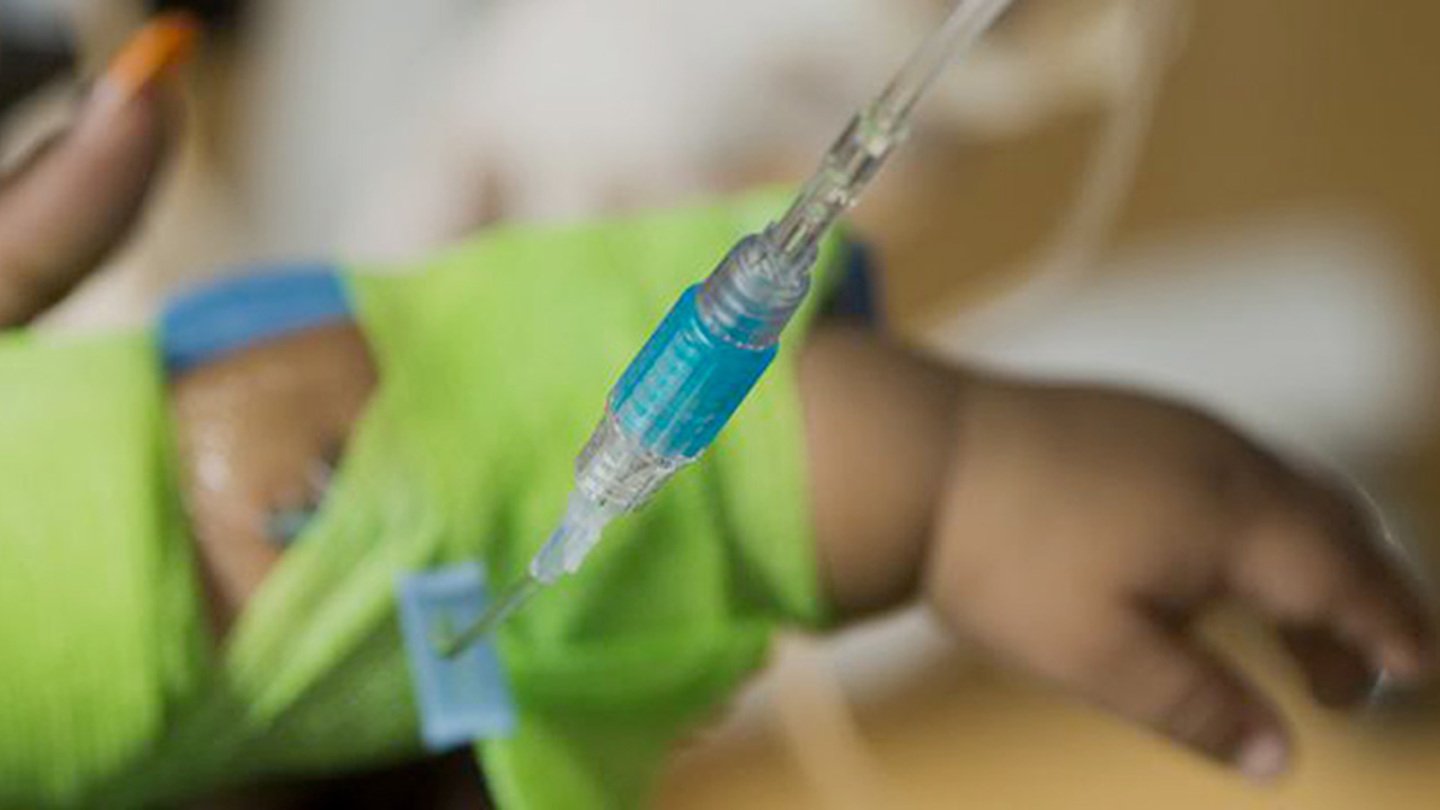
We know that it can be disheartening for a family to learn that their child’s cancer or blood disorder is not responding to treatments or doesn’t have a good standard therapy. The Experimental Therapeutic Program (ETP) team cares for those patients who have recurrent cancer, cancer that hasn't responded to typical treatment or cancers without a known cure. We provide consultation for these patients for possible clinical trials and care for patients being treated on phase one or phase two clinical trials.
We’re tirelessly pursuing new treatment options to improve outcomes and quality of life for all children diagnosed with cancer and blood disorders. Our team provides compassionate, best-in-class care for patients who participate in clinical trials through our program.
About the Experimental Therapeutics Program
As part of the Center for Cancer and Blood Disorders (CCBD) at Children’s Hospital Colorado, our program provides access to some of the world’s top pediatric experts and a wide array of clinical trials.
Many patients with childhood cancer have or will participate in clinical research, sometimes through sharing tumor tissue or sometimes through a treatment trial. The trials available through the Experimental Therapeutics Program study new treatments to learn about the safest dose, the best way to give the treatment and how these treatments work best.
Many of our clinical trials are first-in-pediatrics protocols, meaning they have never been given to children and are only available at a very limited number of hospitals. And, our care goes beyond clinical treatment. We also provide mental and emotional resources for our patients and their families, including our Wellness Program, art therapy and social work.
Our program offers patients access to phase one and phase two cancer and blood disorder clinical trials. The new methods may include:
- New approaches to surgery or radiation
- New methods for gene or cellular therapy
- New drugs or a new combination of existing drugs
- Dosage or strength modification of an existing drug
Our multidisciplinary team
The Experimental Therapeutics Program team includes:
- Board-certified pediatric oncologists, specializing in phase one and phase two trials
- Certified pediatric nurse practitioners and physician assistants
- Experienced oncology research nurses
- Investigational pharmacists
- Research team members who help ensure our research is done safely
- Our Wellness Team, which is available for patients and families in the Center for Cancer and Blood Disorders
In addition to this specialized team, we work with programs throughout our Center for Cancer and Blood Disorders and with the surgical oncology, ortho-oncology and radiation oncology groups as well. This ensures a smooth and efficient transition of patient care and expertise in the patient’s specific disease, whether they come from within our program or across the globe.
Why choose us for experimental therapy care
We are one of a few centers in the United States and Canada that offer and support experimental therapies to patients with relapsed cancer or cancer that is resistant to treatment. In addition to our local and industry partner trials, we are also part of many major cooperative groups and National Cancer Institute-sponsored collaborations.
- Children’s Oncology Group (COG)
- New Agents for Approaches to Neuroblastoma Consortium (NANT)
- Pediatric Brain Tumor Consortium (PBTC)
- CONNECT Consortium for Childhood Brain Tumors
Who we treat at the Experimental Therapeutics Program
Our patients include children, adolescents and young adults who have recurrent cancer, cancer that hasn’t responded to traditional treatment or diagnoses with no known cure. We treat local patients as well as patients from throughout the country and world.
Meet Maelle and learn more about her journey in the program.
Contact the Experimental Therapeutics Program
Our clinical trial options frequently vary over time. For information about trials that might be available, please visit clinicaltrials.gov, email us at ETP@childrenscolorado.org or call 720-777-5771.

Compassionate care, wherever you are
We’re here when you need us. Telehealth appointments are available across every specialty, so you can get the high-quality care we’ve always offered from the comfort, privacy and convenience of home.
See if telehealth is right for you
Get to know our pediatric experts.

Meg Macy, MD
Hematology/Oncology - Pediatric, Pediatrics
Patient ratings and reviews are not available Why?


Kathleen (Katie) Dorris, MD
Hematology/Oncology - Pediatric, Pediatrics




 720-777-0123
720-777-0123





